The Joule-Thomson effect is a fundamental thermodynamic phenomenon that describes the temperature change of a real gas when it is allowed to expand freely at constant enthalpy. This effect has significant implications in various fields, including refrigeration, air conditioning, and the liquefaction of gases. This article will provide an in-depth examination of the Joule-Thomson effect, covering its definition, underlying principles, mathematical formulation, applications, and illustrative explanations to enhance understanding.
1. Definition of the Joule-Thomson Effect
The Joule-Thomson effect refers to the change in temperature of a gas when it expands or is compressed without any heat exchange with the environment (adiabatic process) and while maintaining constant enthalpy. In simpler terms, when a gas is allowed to expand through a valve or a porous plug, it can either cool down or heat up depending on its initial temperature and pressure.
Illustrative Explanation: Imagine a balloon filled with air. If you let the air out slowly through a small hole, the air inside the balloon cools down. This cooling effect is similar to the Joule-Thomson effect, where the gas experiences a temperature change during expansion.
2. Historical Background
The Joule-Thomson effect is named after two scientists, James Prescott Joule and William Thomson (Lord Kelvin), who studied the behavior of gases in the mid-19th century. Joule conducted experiments to understand the relationship between heat and work, while Thomson explored the cooling effect of gas expansion. Their combined work led to the formulation of the Joule-Thomson effect, which is crucial for understanding gas behavior in thermodynamic processes.
Illustrative Explanation: Think of Joule and Thomson as pioneers exploring a new land (the behavior of gases). Joule mapped out the terrain (heat and work), while Thomson discovered hidden valleys (cooling effects) that changed the landscape of thermodynamics.
3. Underlying Principles of the Joule-Thomson Effect
The Joule-Thomson effect is based on the principles of thermodynamics, particularly the concepts of enthalpy, internal energy, and the behavior of real gases. Here are the key principles:
- Enthalpy (H): Enthalpy is a thermodynamic property that represents the total heat content of a system. It is defined as the sum of the internal energy (U) and the product of pressure (P) and volume (V):
![]()
In a Joule-Thomson process, the enthalpy remains constant, meaning that any change in internal energy is balanced by work done on or by the gas.
- Real Gases vs. Ideal Gases: The behavior of real gases deviates from that of ideal gases, especially at high pressures and low temperatures. The Joule-Thomson effect is particularly significant for real gases, as it accounts for intermolecular forces and the volume occupied by gas molecules.
- Intermolecular Forces: The temperature change during the Joule-Thomson effect is influenced by the attractive and repulsive forces between gas molecules. When a gas expands, these forces can either absorb or release energy, leading to a temperature change.
Illustrative Explanation: Imagine a group of friends (gas molecules) at a party (the gas). If they are all standing close together (high pressure), they can feel each other’s warmth (intermolecular forces). When they spread out (expand), some may feel cooler (temperature drop) while others may feel warmer (temperature rise), depending on how they interact.
4. Mathematical Formulation
The Joule-Thomson effect can be quantitatively described using the Joule-Thomson coefficient (![]() ), which indicates the change in temperature (
), which indicates the change in temperature (![]() ) with respect to a change in pressure (
) with respect to a change in pressure (![]() ) at constant enthalpy:
) at constant enthalpy:
![]()
- If
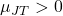 : The gas cools upon expansion (e.g., most gases at room temperature).
: The gas cools upon expansion (e.g., most gases at room temperature). - If
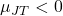 : The gas heats up upon expansion (e.g., hydrogen and helium at room temperature).
: The gas heats up upon expansion (e.g., hydrogen and helium at room temperature).
Illustrative Explanation: Think of the Joule-Thomson coefficient as a thermometer that tells you how a gas will react when you change its pressure. If the thermometer reads positive, the gas will cool down; if it reads negative, the gas will heat up. It’s like a mood ring for gases, indicating their response to pressure changes.
5. Applications of the Joule-Thomson Effect
The Joule-Thomson effect has several practical applications in various fields:
- Refrigeration and Air Conditioning: The Joule-Thomson effect is utilized in refrigeration cycles, where gases are compressed and then allowed to expand, resulting in cooling. This principle is fundamental in the operation of refrigerators and air conditioning systems.
- Gas Liquefaction: The effect is crucial in the liquefaction of gases, such as natural gas and oxygen. By compressing and then expanding these gases, they can be cooled to their liquefaction points, allowing for easier storage and transportation.
- Cryogenics: In cryogenic applications, the Joule-Thomson effect is used to achieve extremely low temperatures. Gases like helium are cooled through expansion, enabling the study of materials and phenomena at cryogenic temperatures.
Illustrative Explanation: Imagine a magician (the refrigeration system) who can make things disappear (cooling). By compressing a gas (making it smaller) and then letting it expand (making it larger), the magician creates a cool breeze (cooling effect) that can chill your drinks or keep your food fresh.
6. Factors Influencing the Joule-Thomson Effect
Several factors influence the Joule-Thomson effect, including:
- Initial Temperature and Pressure: The initial conditions of the gas play a significant role in determining whether it will cool or heat upon expansion. For example, at room temperature, most gases cool upon expansion, while at higher temperatures, some gases may heat up.
- Type of Gas: Different gases exhibit different Joule-Thomson coefficients. For instance, noble gases like helium and neon have negative coefficients at room temperature, meaning they heat up upon expansion, while most other gases cool down.
- Molecular Structure: The molecular structure and intermolecular forces of the gas also affect the Joule-Thomson effect. Gases with stronger intermolecular attractions tend to cool more upon expansion.
Illustrative Explanation: Think of a group of friends at a party again. If the party starts off cool (low temperature), everyone enjoys the atmosphere (most gases cool upon expansion). However, if the party gets too hot (high temperature), some friends might start to feel uncomfortable and want to leave (some gases heat up upon expansion).
7. Experimental Observation of the Joule-Thomson Effect
The Joule-Thomson effect can be experimentally observed using a simple setup:
1. Apparatus: A gas cylinder is connected to a valve and a temperature sensor. The gas is initially at a known pressure and temperature.
2. Expansion: The gas is allowed to expand through the valve into a lower-pressure chamber. The temperature is measured before and after the expansion.
3. Observation: The change in temperature is recorded, and the Joule-Thomson coefficient can be calculated based on the initial conditions and the observed temperature change.
Illustrative Explanation: Imagine conducting a science experiment where you have a balloon filled with air. You measure the temperature of the air inside the balloon before you let it go. Once you release the air, you measure the temperature again. The difference in temperature tells you how the air reacted to the change in pressure, just like observing the Joule-Thomson effect in a gas.
Conclusion
The Joule-Thomson effect is a fundamental thermodynamic phenomenon that describes the temperature change of a gas during expansion or compression at constant enthalpy. By exploring the definitions, historical background, underlying principles, mathematical formulation, applications, influencing factors, and experimental observations, we gain a deeper appreciation for its significance in various fields. From refrigeration and gas liquefaction to cryogenics, the Joule-Thomson effect plays a crucial role in modern technology and scientific research. Understanding this effect is essential for engineers, scientists, and anyone involved in thermodynamics, as it provides valuable insights into the behavior of gases under varying conditions.