The concept of mass number is fundamental in the field of chemistry and nuclear physics. It plays a crucial role in understanding the structure of atoms, the behavior of elements, and the principles of isotopes. This article will provide a detailed exploration of mass number, including its definition, calculation, significance, relationship with atomic number, and illustrative explanations to enhance comprehension.
1. What is Mass Number?
Mass number (symbolized as ![]() ) is defined as the total number of protons and neutrons in the nucleus of an atom. It is a whole number that provides insight into the atomic structure of an element. The mass number is crucial for distinguishing between different isotopes of an element, which have the same number of protons but different numbers of neutrons.
) is defined as the total number of protons and neutrons in the nucleus of an atom. It is a whole number that provides insight into the atomic structure of an element. The mass number is crucial for distinguishing between different isotopes of an element, which have the same number of protons but different numbers of neutrons.
Mathematical Representation
The mass number can be mathematically represented as:
![]()
Where:
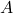 = mass number
= mass number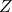 = atomic number (the number of protons)
= atomic number (the number of protons)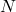 = number of neutrons
= number of neutrons
Illustrative Explanation: Imagine a classroom where students represent protons and neutrons. The total number of students in the classroom (mass number) is the sum of the boys (protons) and girls (neutrons). Just as the total number of students gives you an idea of the classroom size, the mass number provides insight into the atomic structure.
2. Calculating Mass Number
To calculate the mass number of an atom, you need to know the atomic number and the number of neutrons. The atomic number is typically found on the periodic table, while the number of neutrons can be determined by subtracting the atomic number from the mass number.
Example Calculation
Consider the element carbon, which has an atomic number of 6. The most common isotope of carbon, carbon-12, has 6 neutrons. The mass number can be calculated as follows:
![]()
Thus, the mass number of carbon-12 is 12.
Illustrative Explanation: Think of mass number calculation as counting the total number of apples and oranges in a basket. If you have 6 apples (protons) and 6 oranges (neutrons), the total number of fruits (mass number) in the basket is 12.
3. Significance of Mass Number
The mass number is significant for several reasons:
A. Isotopes
Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons, resulting in different mass numbers. For example, carbon has several isotopes, including carbon-12 (![]() ) and carbon-14 (
) and carbon-14 (![]() ). While both isotopes have 6 protons, carbon-12 has 6 neutrons, and carbon-14 has 8 neutrons.
). While both isotopes have 6 protons, carbon-12 has 6 neutrons, and carbon-14 has 8 neutrons.
- Illustrative Explanation: Imagine two different types of fruit smoothies made from the same base ingredients (protons) but with different amounts of additional fruits (neutrons). One smoothie (carbon-12) has 6 strawberries (neutrons), while the other (carbon-14) has 8 strawberries. Both smoothies are still made from the same base, but their flavors (mass numbers) differ.
B. Stability of Nuclei
The mass number is also related to the stability of atomic nuclei. Generally, stable isotopes have a balanced ratio of protons to neutrons. If the number of neutrons is too high or too low compared to the number of protons, the nucleus may become unstable and undergo radioactive decay.
- Illustrative Explanation: Think of a seesaw in a playground. If one side (protons) is too heavy compared to the other side (neutrons), the seesaw will tip over (unstable nucleus). A balanced seesaw represents a stable nucleus, where the mass number reflects a harmonious relationship between protons and neutrons.
C. Chemical Behavior
While the mass number itself does not directly affect the chemical behavior of an element, it is essential for understanding isotopes, which can exhibit different chemical properties under certain conditions. For example, carbon-14 is used in radiocarbon dating, while carbon-12 is the stable form used in organic chemistry.
- Illustrative Explanation: Imagine two friends who are both good at math (the same element) but have different skills in sports (isotopes). One friend (carbon-14) excels in running (radiocarbon dating), while the other (carbon-12) is great at swimming (organic chemistry). Their different skills (properties) arise from their unique experiences (isotopes) despite being the same person (element).
4. Relationship with Atomic Number
The atomic number (symbolized as ![]() ) is the number of protons in the nucleus of an atom and is unique to each element. The relationship between mass number and atomic number is crucial for identifying elements and their isotopes.
) is the number of protons in the nucleus of an atom and is unique to each element. The relationship between mass number and atomic number is crucial for identifying elements and their isotopes.
Notation
The mass number and atomic number are often represented in a specific notation:
![]()
Where:
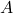 = mass number
= mass number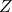 = atomic number
= atomic number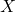 = chemical symbol of the element
= chemical symbol of the element
For example, carbon-12 can be represented as:
![]()
This notation indicates that carbon has a mass number of 12 and an atomic number of 6.
Illustrative Explanation: Think of the notation as a name tag at a conference. The name tag (element symbol) provides essential information about the person (element), while the numbers (mass number and atomic number) give additional details about their background (structure).
5. Applications of Mass Number
Understanding mass number has practical applications in various fields:
A. Nuclear Medicine
In nuclear medicine, isotopes with specific mass numbers are used for diagnostic imaging and treatment. For example, iodine-131 (![]() ) is used to treat thyroid disorders, while technetium-99m (
) is used to treat thyroid disorders, while technetium-99m (![]() ) is commonly used in medical imaging.
) is commonly used in medical imaging.
- Illustrative Explanation: Imagine a toolbox where each tool (isotope) has a specific function. Just as a hammer (iodine-131) is used for driving nails (treating thyroid disorders), a wrench (technetium-99m) is used for tightening bolts (medical imaging). Each tool is chosen based on its unique properties (mass number).
B. Radiocarbon Dating
Radiocarbon dating relies on the presence of carbon-14 to determine the age of organic materials. By measuring the ratio of carbon-14 to carbon-12, scientists can estimate how long it has been since the organism died.
- Illustrative Explanation: Think of radiocarbon dating as a time capsule. The amount of carbon-14 (time marker) in the capsule helps archaeologists determine how long it has been buried (age of the material). The mass number of carbon-14 is crucial for this process.
C. Nuclear Reactions
In nuclear physics, mass number is essential for understanding nuclear reactions, including fission and fusion. The conservation of mass number is a key principle in these processes, ensuring that the total mass number before and after a reaction remains constant.
- Illustrative Explanation: Imagine a game of building blocks. If you start with a certain number of blocks (mass number) and rearrange them to create different structures (nuclear reactions), the total number of blocks remains the same. This principle of conservation is fundamental in nuclear physics.
6. Conclusion
Mass number is a fundamental concept that provides valuable insights into the structure and behavior of atoms. By understanding mass number, we can distinguish between isotopes, assess nuclear stability, and explore the applications of isotopes in various fields, including medicine, archaeology, and nuclear physics. The relationship between mass number and atomic number is crucial for identifying elements and understanding their properties. As we continue to explore the intricacies of atomic structure, the significance of mass number will remain a cornerstone of our understanding of chemistry and physics, connecting us to the very building blocks of matter. Whether in the context of medical applications, dating ancient artifacts, or studying nuclear reactions, mass number serves as a vital tool in our quest to comprehend the universe around us.