Molecular Orbital Theory (MOT) is a fundamental concept in chemistry that provides a framework for understanding the electronic structure of molecules. It describes how atomic orbitals combine to form molecular orbitals, which are spread over the entire molecule rather than being localized between individual atoms. This theory is essential for predicting molecular properties, bonding characteristics, and the behavior of molecules in chemical reactions. This extensive article will delve into the principles of Molecular Orbital Theory, the formation of molecular orbitals, the types of molecular orbitals, and illustrative explanations for each concept.
Definition of Molecular Orbital Theory
Molecular Orbital Theory posits that when atoms combine to form a molecule, their atomic orbitals overlap to create new orbitals known as molecular orbitals. These molecular orbitals can accommodate electrons and are classified based on their energy levels and shapes. MOT provides a more comprehensive understanding of molecular bonding compared to Valence Bond Theory, as it accounts for the delocalization of electrons across the entire molecule.
Illustrative Explanation: Imagine a group of musicians coming together to form a band. Each musician (atomic orbital) contributes their unique sound (electronic characteristics) to create a harmonious piece of music (molecular orbital). The resulting sound is not just a combination of individual instruments but a new, unified performance that represents the collective effort of all musicians.
Formation of Molecular Orbitals
The formation of molecular orbitals occurs through the linear combination of atomic orbitals (LCAO). When two atomic orbitals from different atoms combine, they can do so in two distinct ways:
1. Constructive Interference (Bonding Molecular Orbitals): When two atomic orbitals combine in phase (i.e., their wave functions reinforce each other), they form a bonding molecular orbital. This orbital has lower energy than the original atomic orbitals and is associated with an increased electron density between the nuclei of the bonded atoms, which stabilizes the molecule.
Illustrative Explanation: Picture two waves in the ocean that meet and combine to form a larger wave. This larger wave represents the bonding molecular orbital, where the combined energy of the two waves (atomic orbitals) results in a more stable configuration.
2. Destructive Interference (Antibonding Molecular Orbitals): When two atomic orbitals combine out of phase (i.e., their wave functions cancel each other), they form an antibonding molecular orbital. This orbital has higher energy than the original atomic orbitals and is characterized by a node (a region of zero electron density) between the nuclei, which destabilizes the molecule.
Illustrative Explanation: Imagine two opposing waves in the ocean that meet and cancel each other out, resulting in a flat surface. This flat surface represents the antibonding molecular orbital, where the destructive interference leads to a less stable configuration.
Types of Molecular Orbitals
Molecular orbitals can be classified into two main categories based on their energy levels and bonding characteristics:
1. Bonding Molecular Orbitals: These orbitals are formed from the constructive interference of atomic orbitals and are lower in energy than the original atomic orbitals. Electrons in bonding molecular orbitals contribute to the stability of the molecule.
- Example: In the hydrogen molecule (
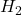 ), the 1s atomic orbitals of two hydrogen atoms combine to form a bonding molecular orbital (
), the 1s atomic orbitals of two hydrogen atoms combine to form a bonding molecular orbital (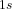 ). This orbital is lower in energy and allows the two hydrogen atoms to bond together.
). This orbital is lower in energy and allows the two hydrogen atoms to bond together.
Illustrative Explanation: Think of bonding molecular orbitals as a strong bridge connecting two islands (atoms). The bridge (orbital) allows for safe passage (electron sharing) between the islands, enhancing stability and connectivity.
2. Antibonding Molecular Orbitals: These orbitals are formed from the destructive interference of atomic orbitals and are higher in energy than the original atomic orbitals. Electrons in antibonding molecular orbitals can weaken or destabilize the bond between atoms.
- Example: In the hydrogen molecule (
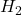 ), the 1s atomic orbitals also combine to form an antibonding molecular orbital (
), the 1s atomic orbitals also combine to form an antibonding molecular orbital (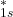 ). This orbital has a node between the nuclei and is higher in energy, indicating that if electrons occupy this orbital, the bond will be weakened.
). This orbital has a node between the nuclei and is higher in energy, indicating that if electrons occupy this orbital, the bond will be weakened.
Illustrative Explanation: Consider antibonding molecular orbitals as a fragile rope connecting two islands. If the rope is too weak (high energy), it may break, leading to a loss of connection (bond) between the islands.
Molecular Orbital Diagrams
Molecular orbital diagrams are graphical representations that illustrate the relative energy levels of molecular orbitals and the distribution of electrons in these orbitals. These diagrams help visualize the bonding and antibonding interactions in a molecule.
1. Filling Molecular Orbitals: Electrons are added to molecular orbitals according to the Aufbau principle, Pauli exclusion principle, and Hund’s rule:
- Aufbau Principle: Electrons fill the lowest energy orbitals first.
- Pauli Exclusion Principle: No two electrons can have the same set of quantum numbers; thus, each orbital can hold a maximum of two electrons with opposite spins.
- Hund’s Rule: When filling degenerate orbitals (orbitals of the same energy), electrons will occupy separate orbitals first before pairing up.
Illustrative Explanation: Imagine a theater with multiple rows of seats (molecular orbitals). The audience (electrons) fills the front rows (lower energy orbitals) first before moving to the back rows (higher energy orbitals). Each seat can hold two people (two electrons), and people prefer to sit alone in a row before sharing a seat.
2. Example of Molecular Orbital Diagram: For diatomic molecules like ![]() (nitrogen), the molecular orbital diagram shows the following:
(nitrogen), the molecular orbital diagram shows the following:
- The
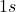 orbitals combine to form
orbitals combine to form 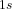 (bonding) and
(bonding) and 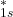 (antibonding).
(antibonding). - The
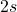 orbitals combine to form
orbitals combine to form 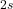 (bonding) and
(bonding) and 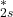 (antibonding).
(antibonding). - The
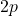 orbitals combine to form
orbitals combine to form 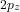 (bonding) and
(bonding) and 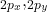 (bonding), as well as
(bonding), as well as 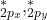 (antibonding).
(antibonding).
Illustrative Explanation: Visualize the molecular orbital diagram as a multi-level parking garage. The lower levels (lower energy orbitals) fill up first, and as they become full, cars (electrons) move to higher levels (higher energy orbitals). The arrangement of cars in the garage reflects the stability and bonding characteristics of the molecule.
Bond Order and Stability
The bond order of a molecule is a measure of the number of chemical bonds between a pair of atoms and is calculated using the formula:
![]()
Where:
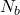 = number of electrons in bonding molecular orbitals
= number of electrons in bonding molecular orbitals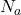 = number of electrons in antibonding molecular orbitals
= number of electrons in antibonding molecular orbitals
A higher bond order indicates a stronger bond and greater stability of the molecule.
Illustrative Explanation: Think of bond order as the strength of a rope bridge connecting two cliffs. A thicker, more robust rope (higher bond order) provides a stronger connection, allowing for safe passage. Conversely, a thin, frayed rope (lower bond order) may not support weight effectively, indicating a weaker bond.
Applications of Molecular Orbital Theory
1. Predicting Molecular Properties: Molecular Orbital Theory allows chemists to predict various properties of molecules, including magnetic behavior, bond lengths, and bond angles. For example, the presence of unpaired electrons in molecular orbitals can indicate whether a molecule is paramagnetic (attracted to a magnetic field) or diamagnetic (not attracted).
Illustrative Explanation: Imagine a team of superheroes, each with unique powers. By analyzing their abilities (molecular properties), you can predict how they will interact with each other and their environment. Similarly, MOT helps chemists understand how molecules will behave based on their electronic structure.
2. Understanding Reaction Mechanisms: MOT provides insights into the mechanisms of chemical reactions by explaining how molecular orbitals change during bond formation and breaking. This understanding is crucial for designing new reactions and materials.
Illustrative Explanation: Consider a dance performance where dancers (molecules) change partners (bonds) throughout the show. By understanding the choreography (reaction mechanisms), you can predict how the performance will unfold and how the dancers will interact.
3. Material Science: Molecular Orbital Theory is essential in material science for designing new materials with specific electronic, optical, and mechanical properties. By manipulating molecular orbitals, scientists can create materials with desired characteristics.
Illustrative Explanation: Think of a sculptor shaping clay into various forms. By understanding the properties of the clay (molecular orbitals), the sculptor can create a masterpiece (new material) with specific features and functions.
4. Biological Systems: MOT is also applicable in biochemistry, where it helps explain the behavior of biomolecules, such as enzymes and DNA. Understanding the electronic structure of these molecules is crucial for elucidating their functions and interactions.
Illustrative Explanation: Imagine a complex machine with many moving parts (biomolecules). By understanding how each part works (molecular orbitals), you can better appreciate how the entire machine operates (biological function).
Conclusion
In conclusion, Molecular Orbital Theory is a powerful framework for understanding the electronic structure of molecules and the nature of chemical bonding. By describing how atomic orbitals combine to form molecular orbitals, MOT provides insights into molecular properties, stability, and reactivity. Key concepts such as bonding and antibonding molecular orbitals, bond order, and molecular orbital diagrams are essential for grasping the principles of this theory. As we continue to explore the intricacies of Molecular Orbital Theory, we can appreciate its significance in chemistry, material science, and biology, ensuring that we utilize this knowledge for the advancement of science and technology. Through ongoing research and education, we can deepen our understanding of this essential concept and its applications, paving the way for innovations and discoveries that benefit humanity.