The particle nature of light is a fundamental concept in physics that describes light as being composed of discrete packets of energy called photons. This duality of light, exhibiting both wave-like and particle-like properties, is central to our understanding of quantum mechanics and the behavior of electromagnetic radiation. This article will delve into the definition of the particle nature of light, its historical development, key concepts, experimental evidence, and illustrative explanations for each aspect.
Definition of the Particle Nature of Light
The particle nature of light refers to the concept that light can be thought of as being made up of particles, specifically photons. Each photon carries a specific amount of energy that is proportional to its frequency, as described by the equation:
![]()
Where:
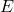 is the energy of the photon.
is the energy of the photon.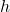 is Planck’s constant (
is Planck’s constant (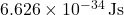 ).
).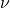 (nu) is the frequency of the light.
(nu) is the frequency of the light.
This particle description complements the wave nature of light, leading to the wave-particle duality concept, which is fundamental in quantum mechanics.
Illustrative Explanation
To visualize the particle nature of light, imagine a stream of tiny balls (photons) being shot from a cannon. Each ball represents a photon, and the speed at which they travel corresponds to the speed of light. Just as the balls can collide with objects and transfer energy, photons can interact with matter, transferring energy in quantized amounts. This analogy helps illustrate how light behaves as a stream of particles while still exhibiting wave-like properties.
Historical Development
The understanding of light as a particle has evolved over centuries, with significant contributions from various scientists:
1. Isaac Newton (1643-1727): Newton proposed the corpuscular theory of light, suggesting that light is made up of particles (corpuscles) that travel in straight lines. He used this theory to explain reflection and refraction.
Illustrative Explanation
Imagine throwing a handful of small balls into a pool. As the balls hit the water, they create ripples (analogous to light waves) but also travel in straight lines. Newton’s idea was that light behaves similarly, traveling in straight paths as particles.
2. Albert Einstein (1879-1955): In 1905, Einstein explained the photoelectric effect, where light shining on a metal surface causes the emission of electrons. He proposed that light consists of photons, each carrying a quantized amount of energy. This work earned him the Nobel Prize in Physics in 1921.
Illustrative Explanation
Picture a basketball player shooting basketballs (photons) at a hoop (metal surface). If the balls are heavy enough (sufficient energy), they can knock the ball out of the hoop (eject electrons). This analogy illustrates how only photons with enough energy can cause the photoelectric effect.
3. Quantum Mechanics: The development of quantum mechanics in the early 20th century further solidified the particle nature of light. The concept of wave-particle duality emerged, showing that light exhibits both wave-like and particle-like behavior depending on the experimental conditions.
Illustrative Explanation
Think of a chameleon that changes color based on its environment. Similarly, light can behave like a wave in some situations (interference patterns) and like a particle in others (photoelectric effect), adapting to the context in which it is observed.
Key Concepts of the Particle Nature of Light
1. Photons: Photons are the fundamental particles of light, characterized by their energy, momentum, and lack of mass. They travel at the speed of light in a vacuum and exhibit both wave-like and particle-like properties.
Illustrative Explanation
Imagine a tiny, massless ball that travels at an incredible speed. This ball represents a photon, moving through space and interacting with other particles. Just as a ball can bounce off a wall, a photon can interact with matter, transferring energy in the process.
2. Energy Quantization: The energy of a photon is quantized, meaning it can only take on specific values determined by its frequency. Higher frequency light (e.g., blue light) has more energy than lower frequency light (e.g., red light).
Illustrative Explanation
Consider a staircase where you can only step on specific steps (quantized energy levels). If you want to reach a higher step (higher energy), you must jump to that level without stopping in between. This analogy illustrates how photons can only possess certain energy levels based on their frequency.
3. Wave-Particle Duality: Light exhibits both wave-like and particle-like behavior, depending on the experimental setup. This duality is a cornerstone of quantum mechanics and is essential for understanding various phenomena.
Illustrative Explanation
Imagine a person wearing two different costumes for a party: one as a wave (flowing and smooth) and the other as a particle (sharp and defined). Depending on the party theme (experimental conditions), the person can switch between the two costumes, just as light can behave as a wave or a particle based on how it is observed.
Experimental Evidence Supporting the Particle Nature of Light
Several key experiments provide compelling evidence for the particle nature of light:
1. Photoelectric Effect: As mentioned earlier, the photoelectric effect demonstrates that light can eject electrons from a metal surface only if the light’s frequency exceeds a certain threshold. This phenomenon cannot be explained by wave theory alone, as it shows that light must consist of discrete packets of energy (photons).
Illustrative Explanation
Picture a game where you need to hit a target with a ball to knock it down. If the ball is too light (low frequency), it won’t knock the target over, regardless of how many times you throw it. However, if you throw a heavier ball (higher frequency), it will knock the target down. This illustrates how only photons with sufficient energy can cause the photoelectric effect.
2. Compton Scattering: In this experiment, X-rays are scattered off electrons, resulting in a change in wavelength. The results can be explained by treating X-rays as particles (photons) colliding with electrons, demonstrating the particle nature of light.
Illustrative Explanation
Imagine two billiard balls colliding on a pool table. When one ball (photon) strikes another (electron), it transfers energy and changes direction. The change in the balls’ motion represents how photons interact with particles, providing evidence for the particle nature of light.
3. Blackbody Radiation: Max Planck’s work on blackbody radiation led to the concept of quantized energy levels, which ultimately supported the idea of light as being composed of photons. Planck’s law describes how the intensity of radiation emitted by a blackbody varies with frequency, reinforcing the particle nature of light.
Illustrative Explanation
Think of a pot of water on a stove. As you heat the water, it reaches specific temperatures (quantized energy levels) before boiling. Similarly, light emitted from a blackbody has specific energy levels based on its frequency, supporting the idea that light consists of particles.
Applications of the Particle Nature of Light
Understanding the particle nature of light has numerous practical applications across various fields:
1. Lasers: Lasers operate based on the principles of stimulated emission, where photons stimulate the emission of more photons, resulting in a coherent beam of light. The particle nature of light is essential for understanding how lasers function.
Illustrative Explanation
Imagine a row of dominoes lined up. When you knock over the first domino (a photon), it causes the next one to fall, and so on. This chain reaction illustrates how photons can stimulate the emission of more photons in a laser.
2. Photovoltaic Cells: Solar panels convert sunlight into electricity through the photoelectric effect. The particle nature of light is crucial for understanding how photons interact with semiconductor materials to generate electrical energy.
Illustrative Explanation
Picture a waterwheel powered by flowing water. As the water (photons) hits the wheel, it turns and generates energy. Similarly, photons hitting a solar cell generate electrical energy by knocking electrons loose.
3. Quantum Computing: The principles of quantum mechanics, including the particle nature of light, are being harnessed in the development of quantum computers. Photons can be used as qubits, the basic units of quantum information.
Illustrative Explanation
Imagine a game of chess where each piece can exist in multiple states at once (superposition). Photons can represent these states in quantum computing, allowing for complex calculations and faster processing.
Conclusion
In conclusion, the particle nature of light is a fundamental concept in physics that describes light as being composed of photons, discrete packets of energy. This understanding has evolved over centuries, with significant contributions from scientists like Newton and Einstein. The particle nature of light is supported by various experimental evidence, including the photoelectric effect and Compton scattering, and has numerous practical applications in fields such as laser technology, solar energy, and quantum computing. By appreciating the intricacies of the particle nature of light, we can better navigate its implications in both scientific and practical contexts, enhancing our understanding of the universe and the behavior of electromagnetic radiation.