Planck’s Quantum Theory, formulated by the German physicist Max Planck in the early 20th century, marks a pivotal moment in the history of physics. It introduced the revolutionary idea that energy is quantized, fundamentally altering our understanding of atomic and subatomic processes. This article will provide a detailed examination of Planck’s Quantum Theory, including its historical context, key concepts, mathematical formulations, implications, and illustrative explanations to enhance comprehension.
1. Historical Context
Definition: The development of Planck’s Quantum Theory arose from the need to explain the phenomenon of blackbody radiation, which classical physics could not adequately describe.
Illustrative Explanation: Imagine a scientist in the late 19th century, akin to a detective trying to solve a mystery. The mystery is the behavior of blackbody radiation, which is the electromagnetic radiation emitted by an idealized perfect black body. Classical theories, like Rayleigh-Jeans law, predicted that the intensity of radiation would increase indefinitely with frequency, leading to what was known as the “ultraviolet catastrophe.” Just as a detective needs new clues to solve a case, physicists needed a new approach to understand this phenomenon.
2. Key Concepts of Planck’s Quantum Theory
A. Quantization of Energy
- Definition: Planck proposed that energy is not continuous but rather comes in discrete packets called “quanta.” The energy of these quanta is proportional to the frequency of the radiation.
- Mathematical Expression: The relationship can be expressed as:
![]()
Where ![]() is the energy of a quantum,
is the energy of a quantum, ![]() is Planck’s constant (
is Planck’s constant (![]() ), and
), and ![]() (nu) is the frequency of the radiation.
(nu) is the frequency of the radiation.
- Illustrative Explanation: Think of energy as a staircase rather than a ramp. Just as you can only step on specific steps (quanta) rather than anywhere on the ramp (continuous energy), energy levels in quantum mechanics are discrete. For example, when an electron in an atom absorbs energy, it can only jump to specific energy levels, akin to stepping up to a higher step on the staircase.
B. Planck’s Constant
- Definition: Planck’s constant is a fundamental physical constant that relates the energy of a photon to its frequency. It is a cornerstone of quantum mechanics.
- Illustrative Explanation: Imagine Planck’s constant as a universal ruler that measures the “size” of energy packets. Just as a ruler helps you understand the dimensions of an object, Planck’s constant helps physicists quantify the energy of photons in the quantum realm.
C. Blackbody Radiation
- Definition: A blackbody is an idealized physical object that absorbs all incident electromagnetic radiation, regardless of frequency or angle of incidence. The radiation emitted by a blackbody is called blackbody radiation.
- Illustrative Explanation: Picture a blackbody as a perfect sponge that soaks up all the water (radiation) that touches it. When heated, it emits radiation in a characteristic spectrum that depends only on its temperature. Planck’s theory provided a formula that accurately described this spectrum, resolving the discrepancies of classical physics.
3. Planck’s Law of Blackbody Radiation
Definition: Planck’s Law describes the spectral density of electromagnetic radiation emitted by a blackbody in thermal equilibrium at a given temperature.
Mathematical Expression: The law can be expressed as:
![]()
Where:
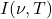 is the spectral radiance,
is the spectral radiance,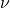 is the frequency,
is the frequency,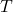 is the absolute temperature,
is the absolute temperature, is the speed of light,
is the speed of light,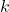 is Boltzmann’s constant.
is Boltzmann’s constant.
Illustrative Explanation: Imagine a campfire glowing in the dark. As you increase the temperature of the fire, it emits light of different colors. Planck’s Law explains how the intensity of light (radiation) changes with temperature and frequency, much like how the color of the fire changes from red to blue as it gets hotter.
4. Implications of Planck’s Quantum Theory
A. Birth of Quantum Mechanics
- Definition: Planck’s Quantum Theory laid the groundwork for the development of quantum mechanics, a fundamental theory in physics that describes the behavior of matter and energy at atomic and subatomic scales.
- Illustrative Explanation: Think of Planck’s theory as the first brick in a new building (quantum mechanics). Just as a building requires a solid foundation, quantum mechanics relies on the principles established by Planck to explore the strange and counterintuitive behaviors of particles.
B. Photoelectric Effect
- Definition: The photoelectric effect is the phenomenon where electrons are emitted from a material when it is exposed to light of sufficient frequency. Albert Einstein later explained this effect using Planck’s concept of quantized energy.
- Illustrative Explanation: Imagine a game of marbles where you need to hit a target with a marble (photon) to knock it down (eject an electron). If the marble doesn’t have enough energy (frequency), it won’t knock the target down, regardless of how many marbles you throw. This illustrates how only photons with sufficient energy can eject electrons from a material.
C. Development of Atomic Models
- Definition: Planck’s Quantum Theory contributed to the development of atomic models, including the Bohr model of the atom, which describes electrons in quantized energy levels.
- Illustrative Explanation: Picture an atom as a solar system, with electrons orbiting the nucleus like planets around the sun. Just as planets can only occupy specific orbits, electrons can only exist in certain energy levels, as dictated by quantum mechanics.
5. Conclusion
In conclusion, Planck’s Quantum Theory represents a monumental shift in our understanding of the physical world. By introducing the concept of quantized energy, Max Planck provided a framework that not only resolved the issues surrounding blackbody radiation but also laid the foundation for the development of quantum mechanics. Through key concepts such as the quantization of energy, Planck’s constant, and the implications for atomic theory and the photoelectric effect, we can appreciate the profound impact of Planck’s work on modern physics. Through illustrative explanations, we can visualize how these concepts interact and contribute to our understanding of the universe at its most fundamental level. Planck’s Quantum Theory continues to influence scientific research and technological advancements, guiding our exploration of the atomic and subatomic realms and shaping the future of physics.