Potassium hydroxide (KOH), commonly known as caustic potash, is a highly versatile and important chemical compound with a wide range of applications in various industries. It is an inorganic compound that is classified as a strong base due to its ability to dissociate completely in water, producing hydroxide ions (OH⁻). This article will provide a detailed exploration of potassium hydroxide, including its properties, production methods, uses, safety considerations, and illustrative explanations to enhance understanding.
1. What is Potassium Hydroxide?
Potassium hydroxide is a white, solid ionic compound that consists of potassium (K) cations and hydroxide (OH⁻) anions. It is highly soluble in water, and its aqueous solution is strongly alkaline. The chemical formula for potassium hydroxide is KOH, and it is often represented in its hydrated form as KOH·H₂O when discussing its interactions with water.
Chemical Structure
The structure of potassium hydroxide can be represented as follows:
![]()
Where:
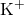 = potassium ion
= potassium ion = hydroxide ion
= hydroxide ion
Illustrative Explanation: Imagine potassium hydroxide as a team of superheroes. The potassium ion (K⁺) is like a strong, supportive hero, while the hydroxide ion (OH⁻) is a quick and agile hero. Together, they form a powerful duo that can tackle various challenges (chemical reactions) effectively.
2. Properties of Potassium Hydroxide
Potassium hydroxide possesses several key properties that make it useful in various applications:
A. Physical Properties
- Appearance: KOH is typically found as a white, crystalline solid or in the form of pellets.
- Solubility: It is highly soluble in water, with a solubility of approximately 121 g per 100 mL at 20°C.
- Melting and Boiling Points: The melting point of potassium hydroxide is around 360°C, and it has a boiling point of approximately 1320°C.
Illustrative Explanation: Think of potassium hydroxide as a sponge. Just as a sponge can absorb a lot of water, KOH can dissolve readily in water, creating a strong alkaline solution that can interact with various substances.
B. Chemical Properties
- Strong Base: KOH is classified as a strong base because it completely dissociates in water, producing a high concentration of hydroxide ions. This property makes it effective in neutralizing acids.
- Reactivity: Potassium hydroxide reacts vigorously with acids to form potassium salts and water. For example, when KOH reacts with hydrochloric acid (HCl), potassium chloride (KCl) and water are produced:
![]()
Illustrative Explanation: Imagine KOH as a firefighter. When it encounters an acid (the fire), it quickly neutralizes it, transforming the dangerous situation (acidic environment) into a safe one (neutral solution).
3. Production of Potassium Hydroxide
Potassium hydroxide can be produced through several methods, with the most common being the electrolysis of potassium chloride (KCl) solution. The process involves the following steps:
A. Electrolysis of Potassium Chloride
1. Preparation: A concentrated solution of potassium chloride is prepared.
2. Electrolysis: The solution is subjected to electrolysis, where an electric current is passed through it. This process causes the KCl to dissociate into potassium ions (K⁺) and chloride ions (Cl⁻).
3. Formation of KOH: At the anode, chloride ions are oxidized to chlorine gas (Cl₂), while at the cathode, potassium ions are reduced to form potassium metal. The potassium metal then reacts with water to produce potassium hydroxide and hydrogen gas (H₂):
![]()
Illustrative Explanation: Think of this process as a relay race. The potassium chloride solution is the starting line, and as the electric current (the baton) is passed through, the potassium ions and chloride ions race to their respective electrodes. The potassium ions reach the finish line (the cathode) and transform into potassium hydroxide, completing the race successfully.
B. Other Methods
Potassium hydroxide can also be produced through the reaction of potassium carbonate (K₂CO₃) with calcium hydroxide (Ca(OH)₂):
![]()
In this reaction, potassium carbonate reacts with calcium hydroxide to yield potassium hydroxide and calcium carbonate (CaCO₃) as a byproduct.
Illustrative Explanation: Imagine this reaction as a cooking recipe. The potassium carbonate is the main ingredient, and when mixed with calcium hydroxide (the cooking agent), it produces a delicious dish (potassium hydroxide) along with a side dish (calcium carbonate).
4. Uses of Potassium Hydroxide
Potassium hydroxide has a wide range of applications across various industries:
A. Agriculture
KOH is used as a fertilizer component, providing potassium, which is essential for plant growth. It helps improve crop yield and quality.
- Illustrative Explanation: Think of potassium hydroxide as a nutrient-rich fertilizer. Just as a gardener adds fertilizer to the soil to help plants grow strong and healthy, KOH provides the necessary potassium for optimal plant development.
B. Manufacturing
Potassium hydroxide is used in the production of soaps, detergents, and cleaning agents. Its strong alkaline properties help in saponification, the process of converting fats and oils into soap.
- Illustrative Explanation: Imagine KOH as a master chef in a kitchen. When combined with oils and fats, it works its magic to create soap, just as a chef combines ingredients to create a delicious dish.
C. Food Industry
In the food industry, potassium hydroxide is used as a food additive and pH regulator. It can be found in products such as olives and pretzels, where it helps maintain the desired texture and flavor.
- Illustrative Explanation: Think of KOH as a flavor enhancer in a recipe. Just as a pinch of salt can elevate the taste of a dish, potassium hydroxide helps improve the quality and safety of food products.
D. Chemical Industry
KOH is used in various chemical processes, including the production of potassium salts, biodiesel, and pharmaceuticals. Its strong basicity makes it a valuable reagent in chemical synthesis.
- Illustrative Explanation: Imagine KOH as a versatile tool in a workshop. Just as a multi-tool can be used for various tasks, potassium hydroxide serves multiple purposes in chemical manufacturing and synthesis.
E. Batteries
Potassium hydroxide is used as an electrolyte in alkaline batteries, where it facilitates the flow of ions and enhances battery performance.
- Illustrative Explanation: Think of KOH as the fuel in a car engine. Just as fuel powers the engine to keep the car running, potassium hydroxide enables the flow of electricity in batteries, ensuring they function effectively.
5. Safety Considerations
While potassium hydroxide is a valuable compound, it is also highly caustic and can pose safety risks if not handled properly. Some safety considerations include:
A. Corrosive Nature
KOH can cause severe burns and irritation to the skin, eyes, and respiratory tract. It is essential to wear appropriate personal protective equipment (PPE), such as gloves, goggles, and masks, when handling this chemical.
- Illustrative Explanation: Imagine KOH as a powerful cleaning agent. Just as you would wear gloves and goggles when using a strong cleaner to protect yourself, the same precautions should be taken when working with potassium hydroxide.
B. Storage and Disposal
Potassium hydroxide should be stored in a cool, dry place, away from incompatible materials such as acids and moisture. Disposal should be conducted according to local regulations, as KOH can have harmful effects on the environment if not disposed of properly.
- Illustrative Explanation: Think of KOH as a valuable treasure. Just as you would store a treasure in a safe place and dispose of it responsibly, potassium hydroxide should be handled with care to prevent accidents and environmental harm.
6. Conclusion
Potassium hydroxide is a versatile and essential chemical compound with a wide range of applications across various industries. Its strong alkaline properties make it valuable in agriculture, manufacturing, food processing, and chemical synthesis. Understanding the properties, production methods, uses, and safety considerations of potassium hydroxide is crucial for its effective and safe application. As we continue to explore the role of potassium hydroxide in our daily lives, we gain a deeper appreciation for its significance in both industrial processes and everyday products. Whether it’s in the fertilizers that nourish our crops or the soaps that keep us clean, potassium hydroxide remains a vital component in the chemistry of life.