Calorimetry is a branch of science that deals with the measurement of heat transfer in physical and chemical processes. It plays a crucial role in various fields, including chemistry, physics, biology, and engineering, by providing insights into energy changes during reactions and phase transitions. The principle of calorimetry is based on the concept of heat exchange, allowing scientists to quantify the energy involved in processes such as combustion, dissolution, and chemical reactions. This article will explore the fundamental principles of calorimetry, the types of calorimeters, the methods used in calorimetry, and illustrative examples to enhance understanding.
What is Calorimetry?
Definition
Calorimetry is the science of measuring the amount of heat absorbed or released during a chemical reaction, physical change, or phase transition. The primary goal of calorimetry is to determine the heat transfer associated with these processes, which can provide valuable information about the energy changes involved.
- Illustrative Explanation: Imagine a sponge soaking up water. The sponge absorbs the water (heat) from its surroundings, and the amount of water it holds can be measured. Similarly, calorimetry measures the heat absorbed or released during a reaction or change.
Basic Concepts in Calorimetry
1. Heat (Q): Heat is a form of energy that is transferred between systems or objects due to a temperature difference. In calorimetry, heat is often measured in joules (J) or calories (cal).
2. Temperature (T): Temperature is a measure of the average kinetic energy of particles in a substance. It is typically measured in degrees Celsius (°C) or Kelvin (K).
3. Specific Heat Capacity (c): Specific heat capacity is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. It is a property of the material and varies from substance to substance.
4. Heat Transfer: Heat transfer occurs when energy moves from a hotter object to a cooler one until thermal equilibrium is reached. In calorimetry, this transfer is crucial for measuring heat changes.
Principle of Calorimetry
The principle of calorimetry is based on the law of conservation of energy, which states that energy cannot be created or destroyed, only transformed from one form to another. In calorimetry, the heat lost by one substance is equal to the heat gained by another substance in a closed system.
Mathematical Representation
The heat transfer can be expressed mathematically as:
![]()
where:
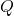 = heat absorbed or released (in joules or calories)
= heat absorbed or released (in joules or calories)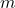 = mass of the substance (in grams)
= mass of the substance (in grams) = specific heat capacity of the substance (in J/g°C or cal/g°C)
= specific heat capacity of the substance (in J/g°C or cal/g°C)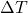 = change in temperature (final temperature – initial temperature, in °C)
= change in temperature (final temperature – initial temperature, in °C)- Illustrative Explanation: Imagine a pot of water on a stove. As the stove heats the pot, the water absorbs heat, causing its temperature to rise. If you know the mass of the water, its specific heat capacity, and the temperature change, you can calculate the total heat absorbed using the formula above.
Heat Exchange in Reactions
In a calorimetric experiment, when a chemical reaction occurs, the heat released or absorbed can be measured. For example, in an exothermic reaction (where heat is released), the system loses heat, while in an endothermic reaction (where heat is absorbed), the system gains heat.
- Illustrative Explanation: Think of a campfire (exothermic reaction) that releases heat into the surrounding air. If you stand close to the fire, you feel warm because the heat is being transferred to you. Conversely, if you put ice in a warm drink (endothermic process), the drink absorbs heat from the surroundings, causing the ice to melt.
Types of Calorimeters
Calorimeters are devices used to measure heat transfer in calorimetry experiments. There are several types of calorimeters, each designed for specific applications:
1. Coffee Cup Calorimeter
A coffee cup calorimeter is a simple, open calorimeter made from two Styrofoam cups, one placed inside the other. It is commonly used for measuring the heat of reaction in aqueous solutions.
- Illustrative Explanation: Imagine two coffee cups stacked together. The inner cup holds the solution, while the outer cup provides insulation. When a reaction occurs in the inner cup, the heat change can be measured by monitoring the temperature change of the solution.
2. Bomb Calorimeter
A bomb calorimeter is a more sophisticated device used to measure the heat of combustion of a substance. It consists of a strong, sealed container (the bomb) that holds the sample and oxygen, surrounded by a water bath.
- Illustrative Explanation: Picture a pressure cooker designed to withstand high temperatures. In a bomb calorimeter, the sample is ignited, and the heat released during combustion raises the temperature of the surrounding water. By measuring this temperature change, the heat of combustion can be calculated.
3. Differential Scanning Calorimeter (DSC)
A differential scanning calorimeter is an advanced instrument used to measure heat flow associated with phase transitions, such as melting and crystallization. It compares the heat flow of a sample with that of a reference material.
- Illustrative Explanation: Imagine two identical pots of water on a stove, one with a lid (the sample) and one without (the reference). As you heat them, the DSC measures the difference in heat flow between the two pots, allowing you to analyze the thermal properties of the sample.
Methods of Calorimetry
Calorimetry can be performed using various methods, depending on the type of reaction or process being studied. The two primary methods are:
1. Constant Pressure Calorimetry
In constant pressure calorimetry, the pressure remains constant throughout the experiment, typically performed in an open system. This method is commonly used for reactions occurring in solutions.
- Illustrative Explanation: Think of a balloon filled with air. As you heat the balloon, the pressure inside remains constant as the air expands. In constant pressure calorimetry, the heat change is measured at constant atmospheric pressure, allowing for accurate calculations of enthalpy changes.
2. Constant Volume Calorimetry
In constant volume calorimetry, the volume of the system remains constant, typically performed in a bomb calorimeter. This method is used for combustion reactions and provides accurate measurements of heat release.
- Illustrative Explanation: Imagine a sealed container filled with gas. When you heat the container, the gas cannot expand, and the pressure increases. In constant volume calorimetry, the heat change is measured at constant volume, allowing for precise calculations of internal energy changes.
Applications of Calorimetry
Calorimetry has numerous applications across various fields, including:
1. Chemical Reactions: Calorimetry is used to determine the heat of reaction for various chemical processes, providing insights into reaction mechanisms and thermodynamics.
2. Biological Processes: In biochemistry, calorimetry is used to study metabolic processes, enzyme activity, and the energy changes associated with biological reactions.
3. Material Science: Calorimetry is employed to analyze phase transitions, such as melting and crystallization, in materials, helping to understand their thermal properties.
4. Food Science: In food chemistry, calorimetry is used to measure the caloric content of food items, providing valuable information for nutrition and dietary planning.
5. Environmental Science: Calorimetry is used to study heat transfer in environmental processes, such as the heat exchange in ecosystems and the thermal properties of soils.
Conclusion
The principle of calorimetry is a fundamental concept in the study of heat transfer and energy changes in physical and chemical processes. By measuring the heat absorbed or released during reactions, calorimetry provides valuable insights into the thermodynamic properties of substances. Understanding the principles of calorimetry, the types of calorimeters, and the methods used in calorimetry is essential for scientists and engineers working in various fields. As technology continues to advance, calorimetry will remain a vital tool for exploring the intricate relationships between energy, heat, and matter, paving the way for innovations in science and industry. By mastering the principles of calorimetry, we can deepen our understanding of the energy changes that govern the natural world and harness this knowledge for practical applications that benefit society.