The quantum theory of light represents a fundamental shift in our understanding of electromagnetic radiation, bridging the gap between classical physics and quantum mechanics. This theory describes light not merely as a wave, as was traditionally understood, but as a dual entity that exhibits both wave-like and particle-like properties. This duality is encapsulated in the concept of photons, the fundamental particles of light. This extensive article will delve into the quantum theory of light, exploring its historical context, key concepts, experimental evidence, and implications, accompanied by illustrative explanations to enhance understanding.
1. Historical Context
The journey toward the quantum theory of light began in the late 19th and early 20th centuries, a period marked by significant advancements in physics. Classical theories, such as Maxwell’s equations, described light as an electromagnetic wave. However, certain phenomena could not be explained by classical wave theory alone, leading to the development of quantum concepts.
- Maxwell’s Equations: Formulated by James Clerk Maxwell, these equations describe how electric and magnetic fields propagate through space, predicting the wave nature of light.
- Blackbody Radiation: The classical theory failed to explain the observed spectrum of radiation emitted by a blackbody (an idealized perfect emitter and absorber of radiation). This discrepancy led to the introduction of quantization.
- Photoelectric Effect: Albert Einstein’s explanation of the photoelectric effect in 1905 provided crucial evidence for the particle nature of light, suggesting that light consists of discrete packets of energy called photons.
Illustrative Explanation: Imagine a grand orchestra (classical physics) playing a symphony (light) where each musician (wave) contributes to the overall sound. However, when the audience (scientists) tries to understand the individual notes (light phenomena), they realize that some notes are played in bursts (photons) rather than as continuous sound waves. This realization prompts a new way of thinking about music (light).
2. Wave-Particle Duality
One of the cornerstone concepts of the quantum theory of light is wave-particle duality, which posits that light exhibits both wave-like and particle-like properties. This duality is essential for understanding various phenomena associated with light.
- Wave Nature: Light behaves as a wave, characterized by properties such as wavelength, frequency, and amplitude. This wave behavior is evident in phenomena like interference and diffraction.
- Particle Nature: Light also behaves as a stream of particles called photons, each carrying a discrete amount of energy given by the equation:
![]()
Where:
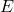 is the energy of the photon,
is the energy of the photon,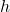 is Planck’s constant (
is Planck’s constant (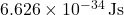 ),
),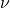 (nu) is the frequency of the light.
(nu) is the frequency of the light.
Illustrative Explanation: Picture a beach (light) where waves (wave nature) crash onto the shore. As you observe the waves, you notice that sometimes they form patterns (interference) and sometimes they seem to hit the sand as individual droplets (photons). This duality illustrates how light can behave like both waves and particles, depending on how you observe it.
3. Photons: The Quantum of Light
Photons are the fundamental particles of light, embodying the particle aspect of the wave-particle duality. Each photon is characterized by its energy, momentum, and polarization.
- Energy and Frequency: The energy of a photon is directly proportional to its frequency. Higher frequency light (e.g., ultraviolet) has more energetic photons than lower frequency light (e.g., infrared).
- Momentum: Photons also carry momentum, which can be observed in phenomena such as radiation pressure. The momentum of a photon is given by:
![]()
Where:
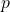 is the momentum,
is the momentum,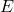 is the energy of the photon,
is the energy of the photon, is the speed of light in a vacuum (
is the speed of light in a vacuum (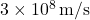 ).
).- Polarization: Photons can be polarized, meaning their electric field oscillates in a specific direction. This property is crucial in applications such as sunglasses and optical filters.
Illustrative Explanation: Imagine a basketball (photon) being thrown across a court (space). The energy of the basketball depends on how fast it is thrown (frequency), and it has a certain momentum based on its speed (momentum). If you spin the basketball while throwing it, it can rotate in a specific direction (polarization), affecting how it interacts with other objects on the court.
4. Quantum Electrodynamics (QED)
Quantum electrodynamics (QED) is the quantum field theory that describes how light and matter interact. It combines the principles of quantum mechanics with the theory of electromagnetism, providing a comprehensive framework for understanding electromagnetic interactions.
- Virtual Particles: In QED, interactions between charged particles (like electrons) and photons are mediated by virtual particles, which are transient fluctuations that occur during interactions. These virtual particles are not directly observable but play a crucial role in calculations.
- Feynman Diagrams: Richard Feynman developed a graphical representation of particle interactions known as Feynman diagrams. These diagrams illustrate the exchange of photons between charged particles, providing a visual tool for understanding complex interactions.
Illustrative Explanation: Think of a busy marketplace (QED) where vendors (charged particles) exchange goods (photons) with each other. Sometimes, the vendors use helpers (virtual particles) to facilitate the exchange, making the process smoother. Feynman diagrams are like maps of the marketplace, showing how goods are traded and how vendors interact.
5. Experimental Evidence
Numerous experiments have confirmed the principles of the quantum theory of light, providing strong evidence for its validity:
- Photoelectric Effect: Einstein’s explanation of the photoelectric effect demonstrated that light can eject electrons from a metal surface only if the light’s frequency exceeds a certain threshold, supporting the particle nature of light.
- Double-Slit Experiment: This classic experiment illustrates wave-particle duality. When light passes through two closely spaced slits, it creates an interference pattern on a screen, demonstrating its wave nature. However, when observed at the level of individual photons, each photon appears to hit the screen as a particle, reinforcing the duality concept.
- Compton Scattering: Arthur Compton’s experiments showed that X-rays scattered off electrons behave as particles, exhibiting a change in wavelength that can be explained by treating light as photons with momentum.
Illustrative Explanation: Imagine a magician (scientist) performing tricks (experiments) that reveal the hidden nature of light. In one trick (photoelectric effect), the magician shows that light can knock over a stack of blocks (electrons) only if it has enough energy (frequency). In another trick (double-slit experiment), the magician creates a beautiful pattern (interference) that changes when the audience tries to peek behind the curtain (observation), revealing the dual nature of light.
6. Implications of the Quantum Theory of Light
The quantum theory of light has profound implications across various fields of science and technology:
- Quantum Computing: The principles of quantum mechanics, including the behavior of photons, are foundational for the development of quantum computers, which leverage quantum bits (qubits) for processing information.
- Laser Technology: The understanding of light as quantized photons is essential for the development of lasers, which have numerous applications in medicine, telecommunications, and manufacturing.
- Photovoltaics: The quantum theory of light underpins the operation of solar cells, which convert light energy into electrical energy through the photoelectric effect.
Illustrative Explanation: Consider a toolbox (quantum theory of light) filled with various tools (technologies) that can be used to build amazing structures (applications). Each tool has its unique function, from the laser (cutting and precision) to the solar panel (capturing energy). Understanding how these tools work allows builders (scientists and engineers) to create innovative solutions for modern challenges.
Conclusion
In conclusion, the quantum theory of light represents a fundamental advancement in our understanding of electromagnetic radiation, encapsulating the dual nature of light as both a wave and a particle. Through the introduction of photons and the development of quantum electrodynamics, this theory has reshaped our comprehension of light-matter interactions and has led to numerous technological advancements. The experimental evidence supporting the quantum theory, including the photoelectric effect, double-slit experiment, and Compton scattering, reinforces its validity and significance. As we continue to explore the intricacies of light and its quantum behavior, we can appreciate the profound impact of the quantum theory of light on science and technology, paving the way for future discoveries and innovations that will continue to enhance our understanding of the universe. Through ongoing research and education, we can deepen our appreciation for the principles established by quantum theory, ultimately contributing to advancements that benefit humanity and expand our knowledge of the natural world.