In the study of gases, the concept of “real gases” refers to gases that do not behave ideally under all conditions. While the Ideal Gas Law provides a useful approximation for many gases at standard temperature and pressure (STP), real gases exhibit deviations from ideal behavior due to intermolecular forces and the finite volume of gas molecules. Understanding the behavior of real gases is crucial for applications in chemistry, engineering, and environmental science. This article will delve into the definition, characteristics, behavior, equations, applications, and limitations of real gases, providing a thorough understanding of this essential concept, complete with illustrative explanations to enhance comprehension.
Definition of Real Gases
Real gases are gases that do not conform to the Ideal Gas Law under all conditions. The Ideal Gas Law, expressed as:
![]()
assumes that gas molecules have no volume and do not exert forces on one another. However, real gases have finite volumes and experience intermolecular attractions and repulsions, leading to deviations from the ideal behavior predicted by the law.
Illustrative Explanation: Imagine a group of friends in a crowded room. If they were to behave like ideal gas molecules, they would move freely without interacting with each other. However, in reality, they bump into each other (intermolecular forces) and take up space (finite volume), which affects how they move around the room.
Characteristics of Real Gases
Real gases exhibit several key characteristics that differentiate them from ideal gases:
1. Intermolecular Forces
Real gases experience intermolecular forces, which can be attractive or repulsive. These forces arise from interactions between gas molecules and can significantly affect gas behavior, especially at high pressures and low temperatures.
- Attractive Forces: These forces pull gas molecules closer together, reducing the volume of the gas compared to what would be predicted by the Ideal Gas Law.
- Repulsive Forces: These forces push gas molecules apart when they are very close to each other, preventing them from occupying the same space.
Illustrative Explanation: Think of a group of friends playing tug-of-war. When they are far apart, they can move freely (ideal behavior). However, as they get closer, they start to pull on each other (attractive forces) and push against each other (repulsive forces), affecting their movement.
2. Finite Volume of Gas Molecules
Real gas molecules occupy a finite volume, which becomes significant at high pressures. As the pressure increases, the volume occupied by the gas molecules themselves cannot be ignored, leading to deviations from ideal behavior.
Illustrative Explanation: Imagine a jar filled with marbles. If you try to fit more marbles into the jar (increasing pressure), you will eventually reach a point where the marbles cannot be compressed any further (finite volume), and the jar will not hold any more marbles.
3. Non-ideal Behavior at High Pressure and Low Temperature
Real gases deviate from ideal behavior primarily under conditions of high pressure and low temperature. At high pressures, the volume of the gas molecules becomes significant, and at low temperatures, intermolecular forces become more pronounced.
Illustrative Explanation: Picture a balloon filled with air. When you squeeze the balloon (high pressure), the air molecules are forced closer together, and their interactions become more significant. If you cool the balloon (low temperature), the air molecules move slower, and the attractive forces between them become more noticeable, causing the balloon to shrink.
Behavior of Real Gases
The behavior of real gases can be described using various equations that account for deviations from ideal behavior. One of the most commonly used equations is the Van der Waals equation, which modifies the Ideal Gas Law to include terms for intermolecular forces and the volume of gas molecules:
![]()
Where:
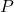 = Pressure of the gas
= Pressure of the gas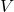 = Volume of the gas
= Volume of the gas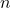 = Number of moles of gas
= Number of moles of gas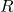 = Ideal gas constant
= Ideal gas constant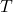 = Absolute temperature in Kelvin
= Absolute temperature in Kelvin = Measure of the attractive forces between gas molecules
= Measure of the attractive forces between gas molecules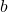 = Volume occupied by one mole of gas molecules
= Volume occupied by one mole of gas molecules
1. The Van der Waals Equation
The Van der Waals equation introduces two constants, ![]() and
and ![]() , which are specific to each gas and account for the effects of intermolecular forces and molecular volume, respectively.
, which are specific to each gas and account for the effects of intermolecular forces and molecular volume, respectively.
- The
 term: This term corrects for the attractive forces between gas molecules. The greater the value of
term: This term corrects for the attractive forces between gas molecules. The greater the value of  , the stronger the intermolecular forces, leading to a greater deviation from ideal behavior.
, the stronger the intermolecular forces, leading to a greater deviation from ideal behavior. - The
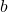 term: This term accounts for the volume occupied by gas molecules. A larger value of
term: This term accounts for the volume occupied by gas molecules. A larger value of 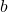 indicates that the gas molecules occupy more space, which also contributes to deviations from ideal behavior.
indicates that the gas molecules occupy more space, which also contributes to deviations from ideal behavior.
Illustrative Explanation: Imagine a group of friends trying to fit into a small car. The car’s size (volume) limits how many friends can fit inside (finite volume), and if they start pushing against each other (intermolecular forces), it becomes even more difficult to fit everyone in. The Van der Waals equation helps quantify these effects.
2. Compressibility Factor (Z)
The compressibility factor ![]() is another important concept used to describe the behavior of real gases. It is defined as:
is another important concept used to describe the behavior of real gases. It is defined as:
![]()
Where:
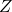 = Compressibility factor
= Compressibility factor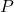 = Pressure of the gas
= Pressure of the gas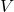 = Volume of the gas
= Volume of the gas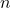 = Number of moles of gas
= Number of moles of gas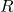 = Ideal gas constant
= Ideal gas constant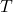 = Absolute temperature in Kelvin
= Absolute temperature in Kelvin
For ideal gases, ![]() . For real gases,
. For real gases, ![]() can be greater than or less than 1, indicating deviations from ideal behavior:
can be greater than or less than 1, indicating deviations from ideal behavior:
 : Indicates that the gas is more compressible than predicted by the Ideal Gas Law, often due to attractive intermolecular forces.
: Indicates that the gas is more compressible than predicted by the Ideal Gas Law, often due to attractive intermolecular forces.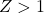 : Indicates that the gas is less compressible than predicted, often due to repulsive intermolecular forces.
: Indicates that the gas is less compressible than predicted, often due to repulsive intermolecular forces.
Illustrative Explanation: Think of a sponge (real gas) compared to a solid block (ideal gas). When you apply pressure to the sponge, it compresses more easily (Z < 1), while the solid block resists compression (Z > 1). The compressibility factor helps quantify these differences.
Applications of Real Gases
Understanding the behavior of real gases is crucial in various fields, including:
1. Chemical Engineering
In chemical engineering, the behavior of real gases is essential for designing reactors, separation processes, and other equipment. Engineers must account for deviations from ideal behavior to ensure efficient and safe operations.
Illustrative Explanation: Imagine a factory producing chemicals. Engineers must design the equipment to handle gases that may not behave ideally, ensuring that reactions occur efficiently and safely, much like a chef adjusting a recipe based on the ingredients available.
2. Environmental Science
Real gases play a significant role in environmental science, particularly in understanding atmospheric gases and their interactions. For example, the behavior of greenhouse gases, such as carbon dioxide and methane, must be studied to assess their impact on climate change.
Illustrative Explanation: Picture the Earth’s atmosphere as a giant greenhouse. Understanding how real gases behave helps scientists predict how these gases will trap heat and affect the planet’s temperature, similar to how a gardener monitors conditions in a greenhouse to optimize plant growth.
3. Refrigeration and Air Conditioning
In refrigeration and air conditioning systems, real gases are used as refrigerants. Understanding their behavior under various conditions is crucial for designing efficient cooling systems.
Illustrative Explanation: Think of a refrigerator as a magic box that keeps your food cold. The refrigerant gas inside must be carefully chosen and managed to ensure it behaves correctly under different temperatures and pressures, much like a conductor leading an orchestra to create harmonious music.
4. Meteorology
Meteorologists study real gases to understand weather patterns and atmospheric phenomena. The behavior of water vapor, carbon dioxide, and other gases is critical for predicting weather changes and climate trends.
Illustrative Explanation: Imagine a weather balloon rising into the atmosphere. As it ascends, the gases inside behave differently based on temperature and pressure changes. Meteorologists use this information to forecast the weather, similar to how a sailor reads the wind to navigate a ship.
Limitations of Real Gases
While the study of real gases is essential, there are limitations to consider:
1. Deviations from Ideal Behavior
Real gases deviate from ideal behavior, especially under high pressure and low temperature. This can complicate calculations and predictions, requiring more complex models to describe their behavior accurately.
Illustrative Explanation: Think of a group of friends trying to fit into a small car. As they get closer together (high pressure), their interactions become more significant, making it difficult to predict how they will move. Similarly, real gases can behave unpredictably under certain conditions.
2. Complexity of Intermolecular Forces
The interactions between gas molecules can be complex and vary significantly between different gases. This complexity makes it challenging to develop a universal model that accurately describes all real gases.
Illustrative Explanation: Imagine a group of friends with different personalities. Some are very friendly (strong attractive forces), while others are more standoffish (weak attractive forces). Understanding how they interact can be complicated, just as it is with different gases.
3. Limited Applicability of Models
While equations like the Van der Waals equation provide valuable insights, they may not accurately predict the behavior of all real gases under all conditions. More sophisticated models may be required for certain gases or conditions.
Illustrative Explanation: Think of a recipe that works well for most cakes but fails for a specific type of cake (real gas). In such cases, a different recipe (model) may be needed to achieve the desired results.
Conclusion
In conclusion, real gases are an essential concept in the study of gas behavior, highlighting the deviations from ideal behavior due to intermolecular forces and the finite volume of gas molecules. By understanding the definition, characteristics, behavior, applications, and limitations of real gases, we gain valuable insights into their implications in various scientific fields. The study of real gases serves as a cornerstone for further research in thermodynamics, physical chemistry, and engineering. As we continue to explore the intricacies of gas behavior, we unlock new possibilities for innovation and discovery, ultimately enriching our understanding of the natural world and its complex chemical processes. Through ongoing research and development, the principles governing real gases will continue to play a vital role in shaping the future of science and technology, contributing to solutions that address global challenges and improve our quality of life.