Chemical reactions are fundamental processes that involve the transformation of substances through the breaking and forming of chemical bonds. Understanding the different types of chemical reactions is essential for students, chemists, and anyone interested in the sciences, as these reactions are the basis for everything from biological processes to industrial applications. This article will explore the main types of chemical reactions, providing detailed explanations and illustrative examples for each category.
1. Synthesis Reactions
Definition: A synthesis reaction, also known as a combination reaction, occurs when two or more reactants combine to form a single product. The general form of a synthesis reaction can be represented as:
![]()
Illustrative Explanation: Imagine a chef combining various ingredients to create a new dish. Just as the chef mixes flour, sugar, and eggs to bake a cake, in a synthesis reaction, different elements or compounds come together to form a new compound.
Example: A classic example of a synthesis reaction is the formation of water from hydrogen and oxygen gases:
![]()
In this reaction, two molecules of hydrogen gas combine with one molecule of oxygen gas to produce two molecules of water.
2. Decomposition Reactions
Definition: A decomposition reaction occurs when a single compound breaks down into two or more simpler products. The general form of a decomposition reaction can be represented as:
![]()
Illustrative Explanation: Think of a puzzle that, when taken apart, reveals its individual pieces. Just as the puzzle breaks down into separate components, a compound in a decomposition reaction splits into simpler substances.
Example: A common example of a decomposition reaction is the breakdown of calcium carbonate (![]() ) when heated:
) when heated:
![]()
In this reaction, calcium carbonate decomposes into calcium oxide and carbon dioxide gas when subjected to heat.
3. Single Replacement Reactions
Definition: A single replacement reaction, also known as a single displacement reaction, occurs when one element replaces another element in a compound. The general form of a single replacement reaction can be represented as:
![]()
Illustrative Explanation: Imagine a game of musical chairs where one player (element) replaces another in a seat (compound). Just as the new player takes the place of the previous one, in a single replacement reaction, one element displaces another in a compound.
Example: An example of a single replacement reaction is the reaction between zinc and hydrochloric acid:
![]()
In this reaction, zinc displaces hydrogen in hydrochloric acid, forming zinc chloride and releasing hydrogen gas.
4. Double Replacement Reactions
Definition: A double replacement reaction, also known as a double displacement reaction, occurs when the ions of two compounds exchange places in an aqueous solution to form two new compounds. The general form of a double replacement reaction can be represented as:
![]()
Illustrative Explanation: Think of a dance where two couples swap partners. Just as the dancers exchange partners to form new pairs, in a double replacement reaction, the ions from two compounds switch places to create new compounds.
Example: A classic example of a double replacement reaction is the reaction between silver nitrate and sodium chloride:
![]()
In this reaction, silver ions and sodium ions exchange places, resulting in the formation of silver chloride and sodium nitrate.
5. Combustion Reactions
Definition: A combustion reaction occurs when a substance reacts with oxygen, releasing energy in the form of light and heat. Combustion reactions typically involve hydrocarbons and can be complete or incomplete, depending on the availability of oxygen. The general form of a complete combustion reaction can be represented as:
![]()
Illustrative Explanation: Imagine a campfire where wood (a hydrocarbon) burns in the presence of oxygen. Just as the fire produces light and heat, a combustion reaction releases energy when a substance reacts with oxygen.
Example: A common example of a combustion reaction is the burning of methane (![]() ):
):
![]()
In this reaction, methane reacts with oxygen to produce carbon dioxide and water, releasing energy in the process.
6. Redox Reactions
Definition: Redox reactions, or oxidation-reduction reactions, involve the transfer of electrons between two species. In these reactions, one species is oxidized (loses electrons) while the other is reduced (gains electrons). The general forms can be represented as:
- Oxidation:
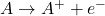
- Reduction:

Illustrative Explanation: Think of a relay race where one runner passes the baton (electrons) to another. Just as the baton is transferred from one runner to another, in a redox reaction, electrons are transferred between reactants.
Example: A classic example of a redox reaction is the reaction between iron and copper(II) sulfate:
![]()
In this reaction, iron is oxidized (loses electrons) while copper(II) ions are reduced (gain electrons), resulting in the formation of iron(II) sulfate and copper metal.
7. Acid-Base Reactions
Definition: Acid-base reactions involve the transfer of protons (H⁺ ions) between reactants. An acid donates a proton, while a base accepts a proton. The general form of an acid-base reaction can be represented as:
![]()
Illustrative Explanation: Imagine a game of catch where one player (the acid) throws a ball (proton) to another player (the base). Just as the ball is passed from one player to another, in an acid-base reaction, protons are transferred between substances.
Example: A common example of an acid-base reaction is the neutralization of hydrochloric acid by sodium hydroxide:
![]()
In this reaction, hydrochloric acid donates a proton to sodium hydroxide, resulting in the formation of sodium chloride (table salt) and water.
Conclusion
Understanding the various types of chemical reactions is essential for grasping the principles of chemistry and the interactions between different substances. From synthesis and decomposition to combustion and redox reactions, each type plays a crucial role in both natural processes and industrial applications. By recognizing the characteristics and examples of these reactions, we can better appreciate the complexity and beauty of chemical transformations that occur all around us. Whether in the laboratory, the environment, or our daily lives, chemical reactions are fundamental to the world we inhabit.