Kohlrausch’s Law, named after the German physicist Friedrich Kohlrausch, is a fundamental principle in the field of electrochemistry that describes the behavior of electrolytes in solution. It provides insights into the conductivity of ionic solutions and the contributions of individual ions to the overall conductivity. This article will delve into the details of Kohlrausch’s Law, including its formulation, significance, applications, and illustrative explanations to enhance understanding.
1. Understanding Conductivity
1.1 Definition of Conductivity
Conductivity is a measure of a solution’s ability to conduct electric current. It depends on the concentration of ions in the solution, the charge of the ions, and their mobility. The higher the concentration of ions, the greater the conductivity.
Illustration: Imagine a crowded highway where cars (ions) are moving. The more cars there are on the road, the easier it is for traffic to flow (electric current). Similarly, a higher concentration of ions leads to increased conductivity.
1.2 Ionic Solutions
Ionic solutions are formed when ionic compounds dissolve in water, dissociating into their constituent ions. For example, when sodium chloride (NaCl) dissolves in water, it separates into sodium ions (Na⁺) and chloride ions (Cl⁻).
Illustration: Picture a bag of marbles (ionic compound) being poured into a pool of water (solvent). As the marbles dissolve, they spread out and become individual balls (ions) floating in the water, allowing for the flow of electricity.
2. Kohlrausch’s Law: Formulation
Kohlrausch’s Law states that the conductivity (κ) of a dilute electrolyte solution can be expressed as the sum of the contributions from each individual ion present in the solution. Mathematically, it can be represented as:
![]()
Where:
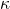 = conductivity of the solution
= conductivity of the solution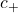 = concentration of the cation
= concentration of the cation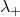 = molar conductivity of the cation
= molar conductivity of the cation = concentration of the anion
= concentration of the anion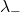 = molar conductivity of the anion
= molar conductivity of the anion
Illustration: Think of a team of athletes (ions) competing in a relay race (conductivity). Each athlete (ion) has a specific speed (molar conductivity) and runs a certain distance (concentration). The total distance covered by the team (conductivity) is the sum of the distances run by each athlete.
3. Molar Conductivity
3.1 Definition
Molar conductivity (![]() ) is defined as the conductivity of an electrolyte solution divided by its molar concentration. It provides a measure of how well an ion can conduct electricity in solution.
) is defined as the conductivity of an electrolyte solution divided by its molar concentration. It provides a measure of how well an ion can conduct electricity in solution.
![]()
Where:
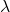 = molar conductivity
= molar conductivity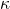 = conductivity of the solution
= conductivity of the solution = concentration of the electrolyte
= concentration of the electrolyte
Illustration: Imagine a water fountain (solution) that sprays water (electricity) into the air. The height of the spray (conductivity) depends on how much water is pumped (concentration) and how efficiently the pump works (molar conductivity).
3.2 Importance of Molar Conductivity
Molar conductivity is crucial for understanding the behavior of ions in solution. It allows chemists to compare the conductive abilities of different ions and to predict how changes in concentration will affect conductivity.
Illustration: Picture a classroom where students (ions) are taking a test (conductivity). Each student has a different level of knowledge (molar conductivity). The overall performance of the class (conductivity) depends on both the number of students (concentration) and their individual knowledge levels.
4. Applications of Kohlrausch’s Law
Kohlrausch’s Law has several important applications in various fields, including:
4.1 Electrochemistry
In electrochemistry, Kohlrausch’s Law is used to determine the molar conductivities of ions, which helps in understanding their behavior in electrochemical cells. This information is vital for designing batteries and fuel cells.
Illustration: Imagine a factory (electrochemical cell) where different machines (ions) work together to produce energy (electricity). Understanding how efficiently each machine operates (molar conductivity) helps engineers optimize the factory’s output.
4.2 Analytical Chemistry
Kohlrausch’s Law is employed in analytical chemistry to analyze the concentration of ions in solution. By measuring the conductivity of a solution, chemists can determine the concentration of specific ions, aiding in quality control and environmental monitoring.
Illustration: Think of a detective (analytical chemist) using a magnifying glass (conductivity measurement) to find clues (ions) in a mystery (solution). The detective’s ability to identify the concentration of clues helps solve the case.
4.3 Environmental Science
In environmental science, Kohlrausch’s Law is used to assess the quality of water by measuring the conductivity of various ions present. High conductivity can indicate pollution or the presence of dissolved salts, which can affect aquatic life.
Illustration: Picture a health inspector (environmental scientist) testing a swimming pool (water body) for cleanliness. The inspector uses a conductivity meter (Kohlrausch’s Law) to determine if the water is safe for swimming, similar to how high conductivity can indicate potential hazards.
5. Limitations of Kohlrausch’s Law
While Kohlrausch’s Law is a powerful tool, it has some limitations:
5.1 Applicability to Dilute Solutions
Kohlrausch’s Law is most accurate for dilute solutions. In concentrated solutions, ion interactions become significant, and the law may not hold true due to deviations caused by ion pairing and other factors.
Illustration: Imagine a crowded concert (concentrated solution) where people (ions) start bumping into each other. The interactions between individuals (ion interactions) can disrupt the flow of the crowd (conductivity), making it difficult to predict movement.
5.2 Ion Mobility Variations
The molar conductivity of ions can vary depending on the solvent and temperature. Changes in these conditions can affect the accuracy of predictions made using Kohlrausch’s Law.
Illustration: Think of a race where the track conditions (solvent and temperature) change. If the track is wet (different solvent), the runners (ions) may not perform as expected, leading to unpredictable results in the race (conductivity).
6. Conclusion
Kohlrausch’s Law is a fundamental principle in electrochemistry that provides valuable insights into the conductivity of ionic solutions. By understanding the contributions of individual ions to overall conductivity, chemists can analyze and predict the behavior of electrolytes in various applications.
From its formulation and significance to its applications and limitations, Kohlrausch’s Law remains a cornerstone of analytical chemistry and electrochemistry. As we continue to explore the complexities of ionic solutions and their behavior, this law serves as a vital tool for scientists and researchers in their quest to understand the world of electrolytes. Whether in the laboratory, industry, or environmental monitoring, Kohlrausch’s Law plays a crucial role in advancing our knowledge of chemical behavior and its practical applications.