Conductivity is a fundamental property of materials that quantifies their ability to conduct electric current. It plays a crucial role in various fields, including electrical engineering, materials science, and environmental science. Understanding conductivity is essential for designing electrical systems, analyzing material properties, and assessing the quality of water and other solutions. This article aims to provide an exhaustive overview of the unit of conductivity, detailing its definition, measurement, factors affecting conductivity, and applications, along with illustrative explanations of each concept.
Understanding Conductivity
1. Definition of Conductivity
Conductivity (![]() ) is defined as the measure of a material’s ability to conduct electric current. It is the reciprocal of resistivity (
) is defined as the measure of a material’s ability to conduct electric current. It is the reciprocal of resistivity (![]() ), which quantifies how strongly a material opposes the flow of electric current. The relationship between conductivity and resistivity is given by the equation:
), which quantifies how strongly a material opposes the flow of electric current. The relationship between conductivity and resistivity is given by the equation:
![]()
Where:
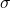 is the conductivity,
is the conductivity,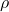 is the resistivity.
is the resistivity.- Illustrative Explanation: Imagine a water pipe. If the pipe is wide and clear (high conductivity), water flows easily through it. If the pipe is narrow and clogged (high resistivity), water struggles to pass through. Conductivity measures how easily electric current can flow through a material, just like how easily water can flow through a pipe.
2. Unit of Conductivity
The standard unit of conductivity in the International System of Units (SI) is siemens per meter (S/m). The siemens (S) is the unit of electrical conductance, which measures how easily electricity flows through a component. One siemens is equivalent to one ampere per volt (A/V).
- Illustrative Explanation: Think of conductivity as a measure of how well a material allows electricity to flow. If a material has a high conductivity (measured in S/m), it means that a large amount of current can flow through it for a given voltage. Conversely, a low conductivity means that less current flows for the same voltage.
3. Other Units of Conductivity
While the SI unit of conductivity is S/m, other units are also used in specific contexts:
- Microsiemens per centimeter (µS/cm): Commonly used in water quality testing, especially for measuring the conductivity of water. One microsiemens is one-millionth of a siemens.
- Millisiemens per centimeter (mS/cm): Used for higher conductivity measurements, such as in saline solutions. One millisiemens is one-thousandth of a siemens.
- Reciprocal ohm (Ω⁻¹): Since conductivity is the reciprocal of resistivity, it can also be expressed in terms of ohms. However, this is less common in practical applications.
- Illustrative Explanation: Imagine measuring the flow of water through different pipes. If you have a very small pipe (like a straw), you might measure the flow in microliters per second. For larger pipes, you might measure in milliliters per second. Similarly, conductivity can be measured in different units depending on the context and the range of values.
Measurement of Conductivity
4. Methods of Measuring Conductivity
Conductivity can be measured using various methods, depending on the material and the application. The most common methods include:
a. Four-Probe Method
This method involves using four electrodes placed in contact with the material. A current is passed through the outer two probes, and the voltage is measured across the inner two probes. This setup minimizes the effects of contact resistance and provides accurate conductivity measurements.
- Illustrative Explanation: Imagine a group of friends trying to measure how fast a river flows. If they stand too close to the bank, they might get splashed (contact resistance). By standing further apart and using a measuring device, they can get a clearer picture of the river’s flow (conductivity).
b. Conductivity Meter
A conductivity meter is a specialized instrument that measures the conductivity of liquids, such as water or chemical solutions. It typically consists of two electrodes submerged in the solution, and it measures the current that flows between them when a voltage is applied.
- Illustrative Explanation: Think of a conductivity meter as a water flow gauge. Just as a gauge measures how much water flows through a pipe, a conductivity meter measures how easily electricity flows through a liquid.
c. Impedance Spectroscopy
This technique involves applying an AC voltage to a material and measuring the resulting current. By analyzing the impedance (the total opposition to current flow), the conductivity can be determined over a range of frequencies.
- Illustrative Explanation: Imagine trying to understand how a crowd moves through a narrow doorway. By observing how quickly people enter and exit at different times (frequencies), you can get a sense of how easily they can pass through (conductivity).
Factors Affecting Conductivity
5. Material Properties
The conductivity of a material depends on its intrinsic properties, such as:
- Type of Material: Metals, such as copper and aluminum, have high conductivity due to the presence of free electrons that can move easily. Insulators, like rubber and glass, have low conductivity because their electrons are tightly bound to their atoms.
- Temperature: For most conductors, conductivity increases with temperature, while for semiconductors and insulators, conductivity typically decreases with increasing temperature.
- Illustrative Explanation: Imagine a crowded room (material) where people (electrons) are trying to move. In a room with wide aisles (high conductivity), people can move freely. In a room with narrow aisles (low conductivity), movement is restricted. As the temperature rises (the room gets warmer), people may become more active and move around more, increasing the overall flow.
6. Concentration of Ions
In solutions, the conductivity is influenced by the concentration of ions present. Higher concentrations of ions lead to higher conductivity because more charge carriers are available to conduct electricity.
- Illustrative Explanation: Think of a saltwater solution. When you dissolve salt in water, the salt dissociates into ions (sodium and chloride). The more salt you add (higher concentration), the more ions are available to carry the electric current, similar to adding more people to a crowded room, making it easier for them to move around.
7. Presence of Impurities
The presence of impurities in a material can significantly affect its conductivity. For example, adding certain impurities to a semiconductor can enhance its conductivity, while impurities in metals can reduce it.
- Illustrative Explanation: Imagine a clear stream (pure material) flowing smoothly. If you introduce rocks and debris (impurities), the flow becomes obstructed, reducing the overall flow rate (conductivity). Conversely, adding certain substances to a semiconductor can create more pathways for current to flow, enhancing conductivity.
Applications of Conductivity
8. Water Quality Testing
Conductivity is a critical parameter in assessing water quality. It provides an indication of the concentration of dissolved salts and minerals in water. High conductivity levels can indicate pollution or high salinity, which can affect aquatic life.
- Illustrative Explanation: Think of a fish tank. If the water is clean and clear (low conductivity), the fish thrive. If the water becomes murky and salty (high conductivity), it can harm the fish. Measuring conductivity helps ensure the water remains healthy for aquatic life.
9. Electronics and Circuit Design
In electronics, conductivity is essential for designing circuits and selecting materials for components. Conductive materials are used for wiring, while insulators are used to prevent unwanted current flow.
- Illustrative Explanation: Imagine building a model train set. You need conductive tracks (high conductivity) for the train to run smoothly and insulators (low conductivity) to keep the electricity from leaking out. Understanding conductivity helps you choose the right materials for your model.
10. Battery Technology
Conductivity plays a vital role in battery performance. The electrolyte’s conductivity affects the battery’s efficiency and charge/discharge rates. Higher conductivity in the electrolyte leads to better battery performance.
- Illustrative Explanation: Think of a battery as a water reservoir. If the water (electricity) can flow easily through the pipes (electrolyte), the battery works efficiently. If the pipes are clogged (low conductivity), the battery struggles to deliver power.
11. Semiconductor Manufacturing
In semiconductor manufacturing, controlling conductivity is crucial for creating devices like transistors and diodes. Doping (adding impurities) is used to modify the conductivity of semiconductors, allowing for the creation of p-type and n-type materials.
- Illustrative Explanation: Imagine a chef adjusting a recipe. By adding different spices (impurities), the chef can change the flavor (conductivity) of the dish. Similarly, by doping semiconductors, manufacturers can tailor their electrical properties for specific applications.
Conclusion
In conclusion, conductivity is a fundamental property that quantifies a material’s ability to conduct electric current. The unit of conductivity, siemens per meter (S/m), provides a standardized way to measure this property. Understanding conductivity, its measurement, factors affecting it, and its applications is essential for various fields, including water quality testing, electronics, battery technology, and semiconductor manufacturing. As technology continues to advance, the study of conductivity will remain crucial for developing innovative solutions and enhancing our understanding of electrical systems and materials.