The Schottky defect is a type of point defect that occurs in ionic crystals, characterized by the absence of an equal number of cations and anions from the crystal lattice. This defect is named after the German physicist Walter Schottky, who contributed significantly to the understanding of defects in solid-state physics. Schottky defects play a crucial role in determining the physical properties of ionic solids, including their electrical conductivity, density, and thermal properties. This article aims to provide a detailed overview of Schottky defects, including their formation, characteristics, implications, and illustrative explanations of each concept to enhance understanding.
Definition of Schottky Defect
What is a Schottky Defect?
A Schottky defect is a type of vacancy defect in an ionic crystal where an equal number of cations and anions are missing from the lattice. This results in a disruption of the regular arrangement of ions in the crystal structure, leading to changes in the material’s properties. The formation of Schottky defects is a thermally activated process, meaning that higher temperatures can increase the likelihood of these defects occurring.
Illustrative Explanation
To visualize a Schottky defect, imagine a perfectly arranged grid of colored tiles representing an ionic crystal. Each tile represents an ion, with one color representing cations (positive ions) and another color representing anions (negative ions). If you were to remove one tile of each color from the grid, you would create a gap in the arrangement. This gap represents a Schottky defect, where the absence of both a cation and an anion disrupts the regular pattern of the crystal.
Formation of Schottky Defects
Mechanism of Formation
Schottky defects form when thermal energy is sufficient to overcome the energy barrier required to remove ions from their lattice positions. The process can be summarized in the following steps:
1. Thermal Excitation: At elevated temperatures, some ions gain enough thermal energy to break free from their lattice sites.
2. Vacancy Creation: When a cation and an anion leave their positions, they create vacancies in the crystal lattice. The number of cation vacancies must equal the number of anion vacancies to maintain charge neutrality.
3. Equilibrium: The concentration of Schottky defects reaches an equilibrium based on temperature and the material’s properties.
Illustrative Explanation
Imagine a crowded dance floor (the crystal lattice) where dancers (ions) are moving in a synchronized manner. As the music (temperature) gets louder, some dancers gain enough energy to step off the dance floor (leave their lattice positions). If one dancer of each type (cation and anion) steps off, a gap is created in the formation. This gap represents a Schottky defect, where the dance floor is no longer perfectly filled.
Characteristics of Schottky Defects
1. Charge Neutrality
One of the defining features of Schottky defects is that they maintain charge neutrality in the crystal. For every cation vacancy created, an anion vacancy must also be created. This balance is crucial for the stability of the ionic crystal structure.
2. Concentration and Temperature Dependence
The concentration of Schottky defects increases with temperature. As the temperature rises, more ions gain sufficient energy to leave their lattice positions, leading to a higher number of vacancies. The relationship between temperature and defect concentration can be described by the Arrhenius equation:
![]()
Where:
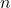 is the concentration of defects.
is the concentration of defects.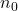 is a pre-exponential factor.
is a pre-exponential factor.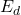 is the energy required to form a defect.
is the energy required to form a defect.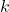 is the Boltzmann constant.
is the Boltzmann constant.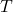 is the absolute temperature.
is the absolute temperature.
3. Impact on Properties
The presence of Schottky defects can significantly affect the physical properties of ionic solids, including:
- Electrical Conductivity: Schottky defects can enhance ionic conductivity by providing pathways for ion migration.
- Density: The formation of vacancies reduces the overall density of the material.
- Thermal Properties: The presence of defects can influence thermal conductivity and heat capacity.
Illustrative Explanation
Think of Schottky defects as holes in a sponge (the ionic crystal). The sponge is normally full of water (ions), but as the temperature rises, some water molecules (ions) evaporate, leaving behind holes (vacancies). The more water that evaporates, the more holes appear, affecting the sponge’s overall structure and properties, such as its ability to hold more water (conductivity) and its weight (density).
Implications of Schottky Defects
1. Ionic Conductivity
Schottky defects play a crucial role in the ionic conductivity of materials, particularly in solid electrolytes used in batteries and fuel cells. The presence of vacancies allows for easier movement of ions through the lattice, enhancing conductivity.
2. Material Stability
The formation of Schottky defects can influence the stability of ionic crystals. While a certain concentration of defects can enhance properties like conductivity, excessive defects may lead to structural instability and degradation of the material.
3. Applications in Technology
Understanding Schottky defects is essential in various technological applications, including:
- Solid-State Batteries: The performance of solid electrolytes is closely related to the concentration of Schottky defects.
- Ceramics: The properties of ceramic materials, such as dielectric constants and mechanical strength, can be influenced by the presence of defects.
- Semiconductors: In semiconductor materials, defects can affect electronic properties and carrier mobility.
Illustrative Explanation
Imagine a busy highway (ionic conductivity) where cars (ions) travel. If there are a few potholes (Schottky defects) on the road, cars can navigate around them, allowing for smoother traffic flow. However, if too many potholes appear, the road becomes difficult to drive on, leading to traffic jams (reduced stability). Understanding the balance of potholes is crucial for maintaining an efficient highway system.
Conclusion
In conclusion, the Schottky defect is a fundamental concept in solid-state physics and materials science, representing a type of point defect characterized by the absence of cations and anions in an ionic crystal lattice. Understanding the formation, characteristics, and implications of Schottky defects is essential for predicting and manipulating the properties of ionic solids. Through illustrative explanations and practical examples, we can appreciate the significance of Schottky defects in various applications, from solid-state batteries to ceramics and semiconductors. As we continue to explore the intricacies of materials science, mastering the concepts surrounding Schottky defects will empower researchers and engineers to design and optimize materials for a wide range of technological advancements.