Pressure is a fundamental physical quantity that describes the force exerted per unit area on a surface. It is a crucial concept in various scientific fields, including physics, engineering, meteorology, and medicine. Understanding pressure is essential for analyzing fluid behavior, designing structures, and studying atmospheric phenomena. This article aims to provide an exhaustive overview of pressure, detailing its definition, units of measurement, factors affecting pressure, applications, and illustrative explanations of each concept.
Understanding Pressure
1. Definition of Pressure
Pressure is defined as the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Mathematically, pressure (![]() ) can be expressed with the formula:
) can be expressed with the formula:
![]()
Where:
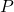 is the pressure,
is the pressure,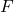 is the force applied,
is the force applied,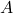 is the area over which the force is distributed.
is the area over which the force is distributed.- Illustrative Explanation: Imagine a person standing on a soft surface, like a beach ball. If the person stands on the ball with both feet, the force exerted by their weight is distributed over the area of both feet. If the person stands on one foot, the same weight is now concentrated on a smaller area, increasing the pressure on that part of the ball, which may cause it to deform more than when both feet are used.
2. Units of Pressure
Pressure can be expressed in various units, depending on the system of measurement used. The most common units of pressure include:
a. SI Units
In the International System of Units (SI), the unit of pressure is the pascal (Pa). One pascal is defined as one newton of force applied over an area of one square meter:
![]()
Where:
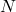 is the newton, the SI unit of force,
is the newton, the SI unit of force,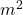 is the square meter, the SI unit of area.
is the square meter, the SI unit of area.- Illustrative Explanation: If a weight of 1 newton is applied to an area of 1 square meter, the pressure exerted is 1 pascal. To visualize this, think of a small book resting on a table. If the book weighs 1 newton and has a surface area of 0.1 m², the pressure it exerts on the table is:
![]()
b. CGS Units
In the centimeter-gram-second (CGS) system, pressure is often measured in dynes per square centimeter (dyn/cm²) or in bars. One bar is defined as 100,000 pascals (Pa).
- Illustrative Explanation: If you have a pressure of 1 bar, it means that the force of 100,000 dynes is applied over an area of 1 cm². This unit is commonly used in meteorology and engineering.
c. Other Units
Pressure can also be expressed in other units, such as atmospheres (atm), millimeters of mercury (mmHg), and pounds per square inch (psi). These units are often used in specific applications:
- 1 atm is approximately equal to 101,325 Pa.
- 1 mmHg is approximately equal to 133.322 Pa.
- 1 psi is approximately equal to 6894.76 Pa.
- Illustrative Explanation: When measuring blood pressure, it is often expressed in mmHg. A reading of 120 mmHg means that the pressure exerted by the blood column is equivalent to the pressure exerted by a column of mercury that is 120 millimeters high.
3. Conversion Between Units
Understanding how to convert between different units of pressure is essential for practical applications. Here are some common conversions:
- From pascals to atmospheres:
![]()
– Example: To convert 202,650 Pa to atm:
![]()
- From mmHg to pascals:
![]()
– Example: To convert 760 mmHg to Pa:
![]()
- Illustrative Explanation: If you have a pressure reading of 30 psi and want to convert it to pascals, you would multiply by 6894.76 Pa/psi, resulting in approximately 206,843 Pa.
Factors Affecting Pressure
4. Force and Area
The pressure exerted on a surface is directly proportional to the force applied and inversely proportional to the area over which the force is distributed. Increasing the force increases the pressure, while increasing the area decreases the pressure.
- Illustrative Explanation: Consider a sharp nail and a flat piece of wood. When you press down on the nail with a certain force, the small area of the nail’s tip concentrates that force, resulting in high pressure that can penetrate the wood. In contrast, if you press down with the same force on the flat piece of wood, the pressure is much lower because the area is larger.
5. Fluid Dynamics
In fluids, pressure can vary with depth due to the weight of the fluid above. This principle is described by Pascal’s law, which states that pressure applied to a confined fluid is transmitted undiminished in all directions.
- Illustrative Explanation: Imagine a swimming pool. The deeper you go, the greater the pressure you feel due to the weight of the water above you. If you dive to a depth of 10 meters, the pressure at that depth is significantly higher than at the surface because of the water column above.
6. Temperature
Temperature can also affect pressure, particularly in gases. According to the ideal gas law, if the volume of a gas is held constant, increasing the temperature will increase the pressure. This relationship is described by Gay-Lussac’s law.
- Illustrative Explanation: Think of a sealed, rigid container filled with gas. If you heat the container, the gas molecules gain kinetic energy and move faster, colliding with the walls of the container more frequently and with greater force. This results in an increase in pressure.
Applications of Pressure
7. Engineering and Construction
Pressure is a critical factor in engineering and construction, particularly in the design of structures, pipelines, and pressure vessels. Engineers must consider the pressure exerted by fluids and gases to ensure safety and structural integrity.
- Illustrative Explanation: When designing a water supply system, engineers must calculate the pressure required to deliver water to various locations. If the pressure is too low, water may not reach higher floors in a building, while excessive pressure could damage pipes.
8. Meteorology
In meteorology, atmospheric pressure is a key factor in weather prediction and analysis. Changes in atmospheric pressure can indicate weather patterns, such as the approach of a storm or the formation of high-pressure systems.
- Illustrative Explanation: A barometer measures atmospheric pressure. A sudden drop in pressure may signal an approaching storm, while a steady increase in pressure often indicates fair weather.
9. Medicine
In medicine, pressure measurements are crucial for diagnosing and monitoring health conditions. Blood pressure, for example, is a vital sign that indicates the force of blood against the walls of blood vessels.
- Illustrative Explanation: A sphygmomanometer is used to measure blood pressure. A reading of 120/80 mmHg indicates the pressure during heartbeats (systolic) and between beats (diastolic), providing important information about cardiovascular health.
10. Fluid Mechanics
In fluid mechanics, pressure is essential for understanding the behavior of fluids in motion. Engineers and scientists analyze pressure changes to design efficient systems for fluid transport, such as pumps and turbines.
- Illustrative Explanation: When designing a water pump, engineers must consider the pressure required to move water through pipes and against gravity. Understanding pressure dynamics helps optimize pump performance and energy efficiency.
Conclusion
In conclusion, the unit of pressure is a vital concept in understanding the physical properties of fluids and gases and their applications across various fields. Pressure quantifies the force exerted per unit area, with different units used in different contexts. Factors such as force, area, fluid dynamics, and temperature significantly influence pressure, making it essential for engineers, scientists, and medical professionals to consider these factors in their work. From engineering and meteorology to medicine and fluid mechanics, pressure plays a crucial role in ensuring the efficiency and effectiveness of processes and products. By comprehensively understanding pressure and its units, we can better appreciate the complexities of the physical world and its impact on our daily lives.